Precision HRD Testing to Uncover PARP Inhibitor Opportunities
One Test. Three Insights —— HRD Score, BRCA1/2, and HRR Gene Alterations.
Applicable to Key Solid Tumors with HRD Relevance
Ovarian, breast, pancreatic, and prostate cancers.
In clinical practice, we often see moments of regret:
A patient with ovarian cancer responds well to first-line platinum-based chemotherapy—only to experience recurrence within months. It makes us wonder:Had a PARP inhibitor been used earlier, could it have reduced her risk of relapse?
Genecast HRD (Homologous Recombination Deficiency) was developed to answer that very question.
Developed by Genecast, Genecast HRD is a comprehensive HRD testing solution that assesses genomic instability, BRCA1/2 mutations, and key alterations in the HRR pathway. By identifying patients with HRD-positive tumors during remission, We can identify patients with higher potential benefit from PARP inhibitor maintenance.
For clinicians, Genecast HRD offers a deeper molecular lens to guide treatment decisions.
For patients, it may open the door to targeted therapies they never knew were possible.
In the journey of precision oncology, Genecast HRD helps us see more—and act sooner.
Why HRD Testing?
HRD is a key predictive biomarker for PARP inhibitor efficacy—not limited to BRCA mutations. With HRD testing, clinicians can:
Expand treatment opportunities beyond BRCA mutations
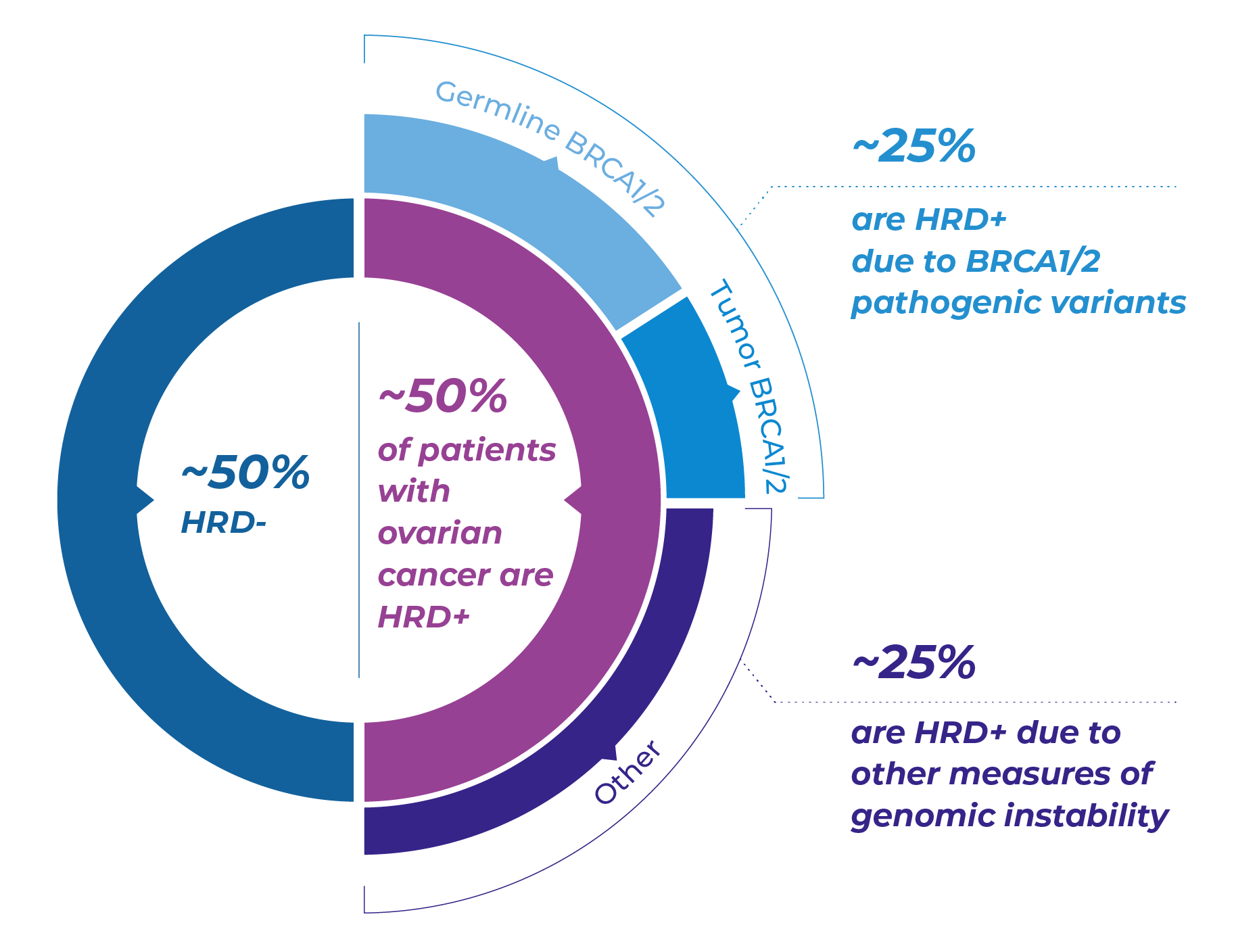
Better identify patients for PARPi maintenance therapy
Delay recurrence and improve progression-free survival. Patients with BRCA mutations or HRD-positive tumors tend to be more sensitive to platinum-based chemotherapy and PARP inhibitors, with improved progression-free survival and overall survival.
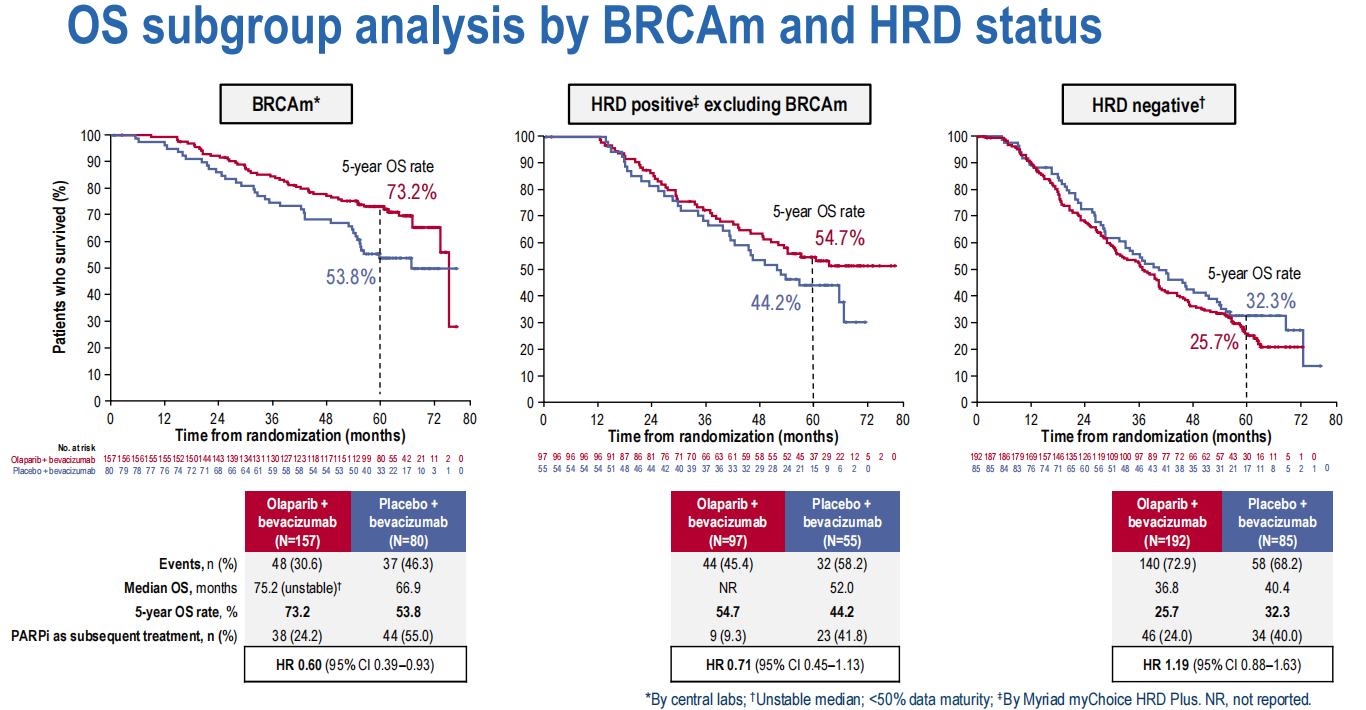
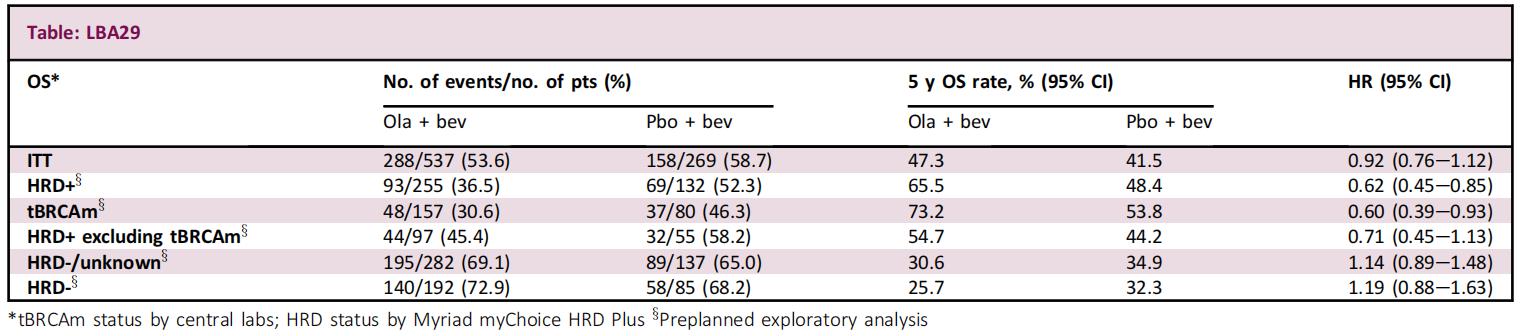
LBA29 Final overall survival (OS) results from the phase III PAOLA-1/ENGOT-ov25 trial evaluating maintenance olaparib (ola) plus bevacizumab (bev) in patients (pts) with newly diagnosed advanced ovarian cancer (AOC)
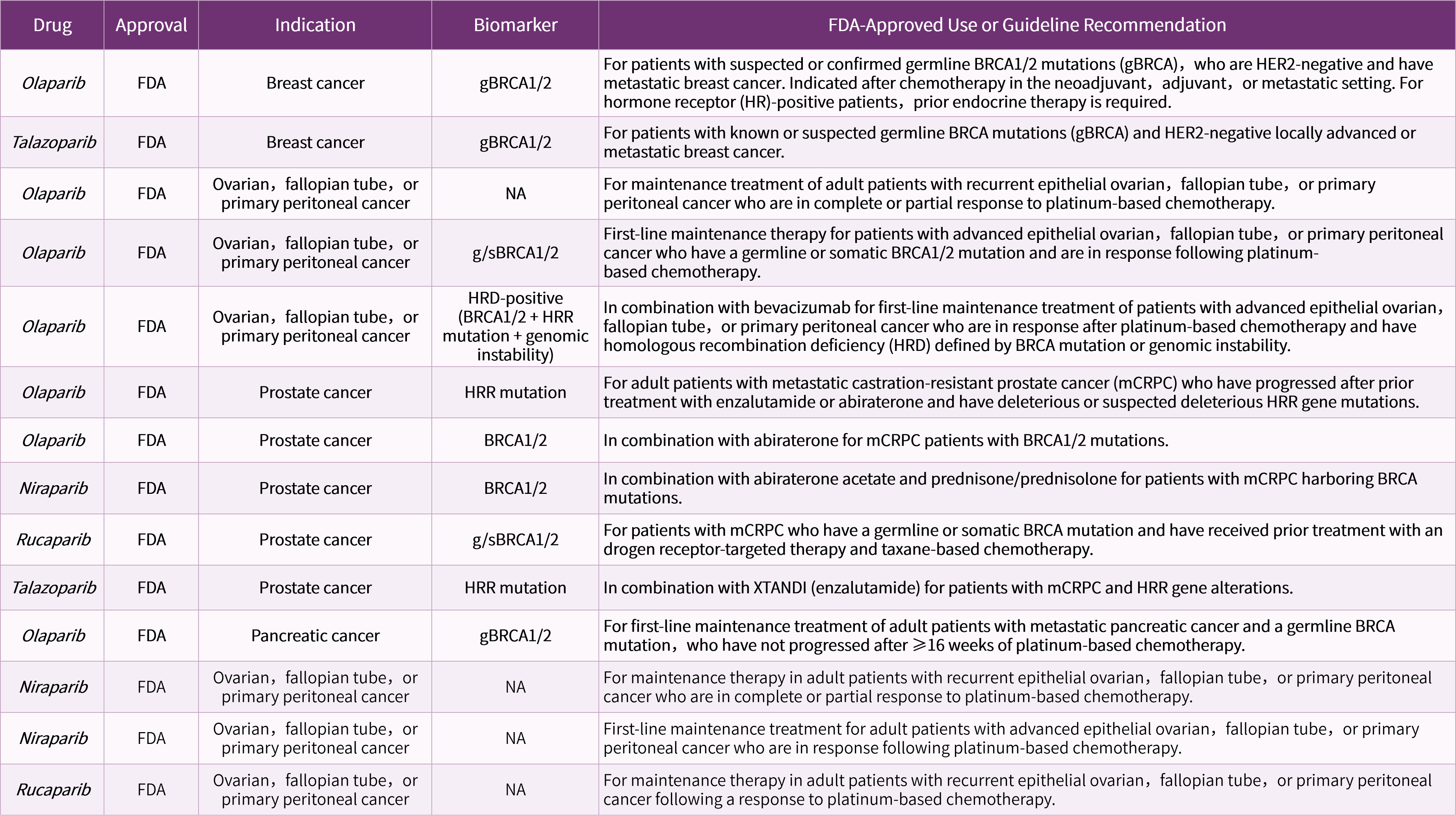
Align with clinical guidelines, including NCCN and ESMO recommendations
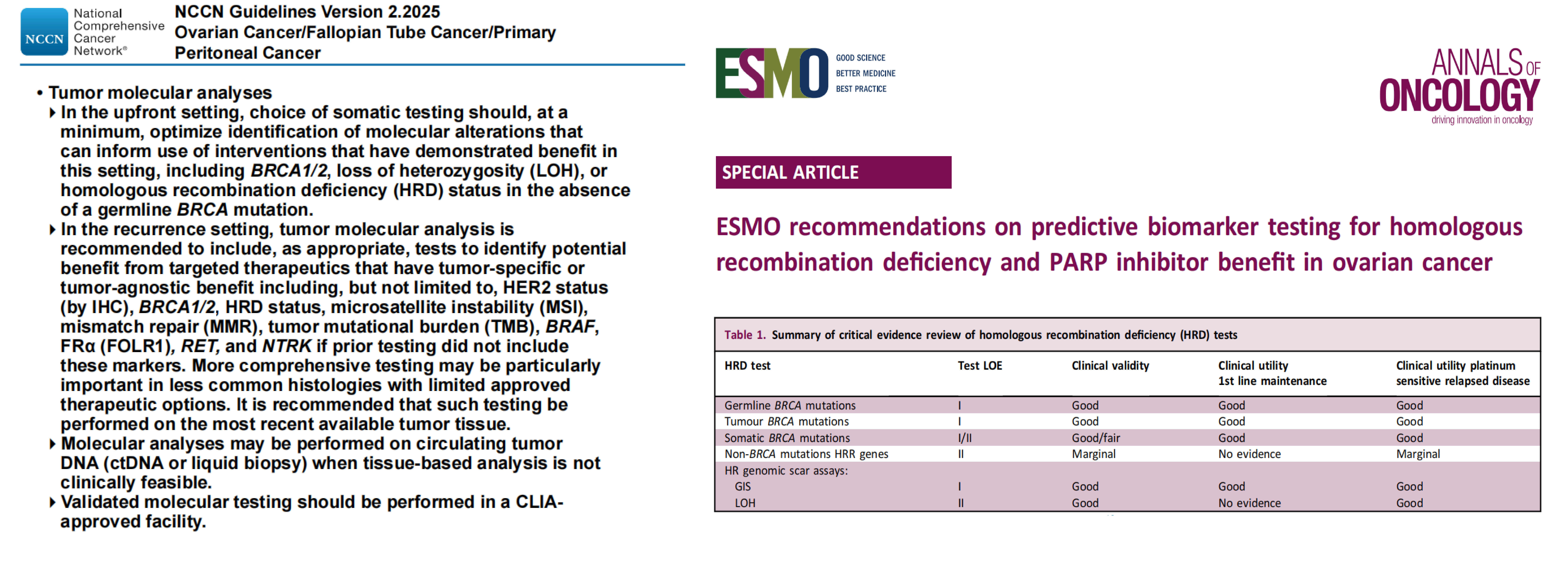
ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann Oncol. 2020 Dec;31(12):1606-1622.
NCCN Guidelines Version 2.2025Ovarian Cancer/Fallopian Tube Cancer/PrimaryPeritoneal Cancer
Why choose Genecast HRD?
Three Key Dimensions. One Comprehensive Solution.
Genecast HRD integrates all critical biomarkers for identifying HRD status and guiding PARP inhibitor use:
1、HRD Score: Based on LOH, TAI, and LST genomic scars
2、BRCA1/2 Mutations: Germline and somatic, including large rearrangements
3、HRR Pathway Genes: Multi-gene panel for broader patient stratification
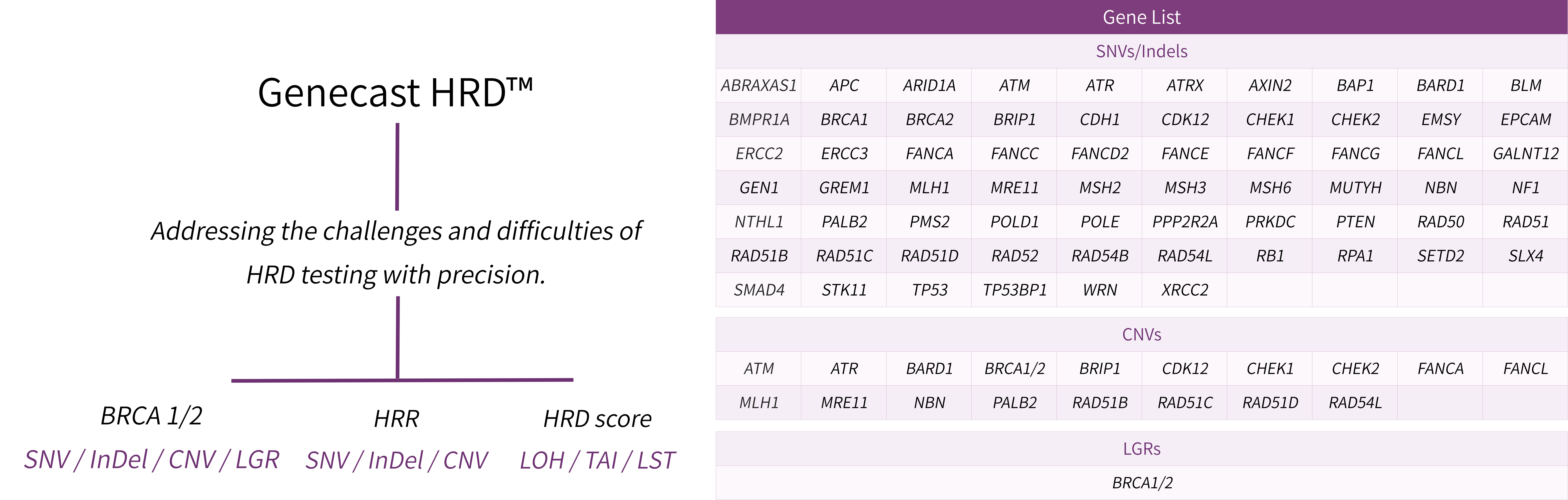
Dual-platform strategy:Integrates low pass WGS and a SNP panel to enhance result stability.
Low tumor requirement: Detects with as little as 20% tumor content, making testing accessible to more patients.
High algorithm accuracy: Achieves 96% consistency between HRD score and BRCAness phenotype.
In addition, Genecast Comprehensive or Genecast Focus can be selected to provide comprehensive guidance on treatment options for patients.
Target Popluation
Patients with newly diagnosed ovarian, breast, pancreatic, or prostate cancer, especially those being considered for platinum-based chemotherapy or PARP inhibitors
Individuals with recurrent or platinum-sensitive ovarian cancer being evaluated for maintenance therapy
Patients with advanced solid tumors (e.g., endometrial, gastric) where genomic instability may guide enrollment in clinical trials or off-label PARPi treatment
Those with strong family history or clinical suspicion of hereditary cancer syndromes
Patients whose treatment plan would benefit from HRD score and mutations in HRR genes (e.g., BRCA1/2, ATM, RAD51, PALB2)
Testing Workflow & Ordering Process
Genecast HRD affordable results in 4-7 business days*
*Upon receipt of tumor specimen
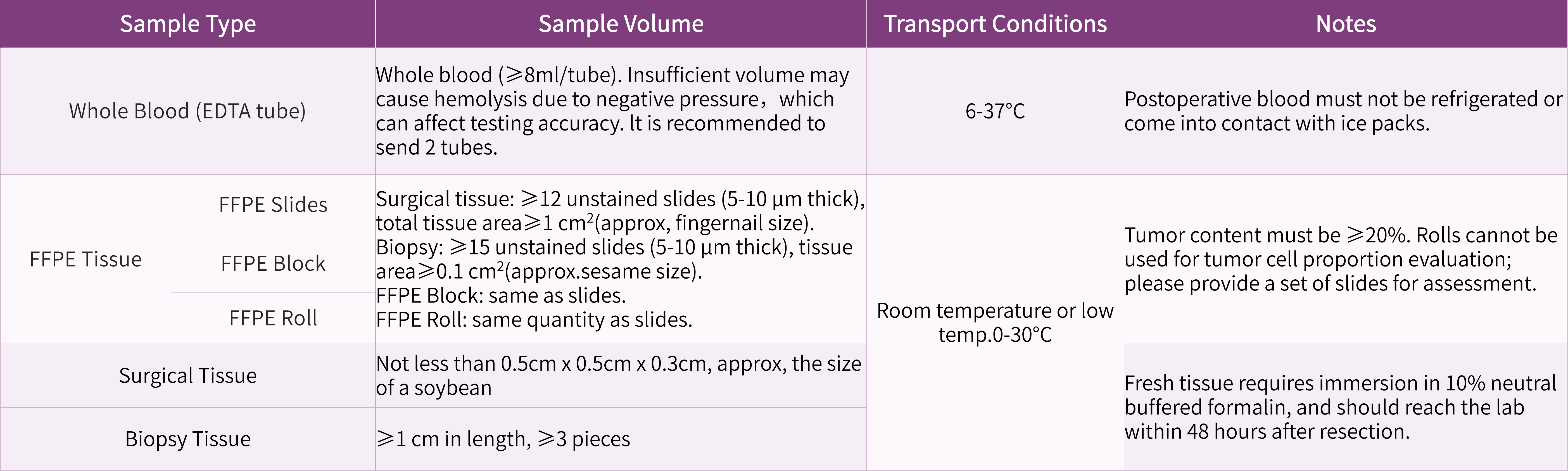
Sample requirements