Multi-Cancer Early Detection. One tube of Blood, One Step Ahead.
Powered by cfDNA whole-methylome multimodal analysis for precise identification of early cancer signals.
Why Is Multi-Cancer Early Detection Important?
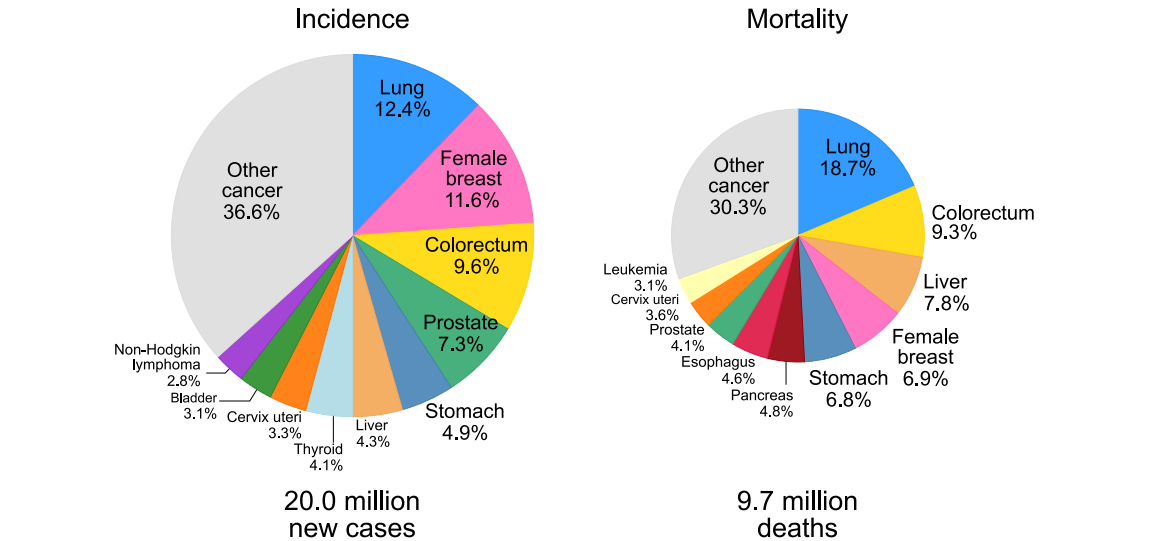
Cancer is now one of the leading causes of death worldwide. Unfortunately, most cancers are diagnosed at advanced stages, when treatment options are limited and outcomes are poor. In contrast, detecting cancer early significantly improves the chances of successful treatment—and in many cases, a cure.
Traditional screening methods focus on individual cancers, such as breast or colorectal cancer. Multi-cancer early detection (MCED) offers a new paradigm: with a single blood draw, multiple high-mortality cancers can be assessed simultaneously, improving detection coverage and clinical efficiency.
Cancer is now one of the leading causes of death worldwide.
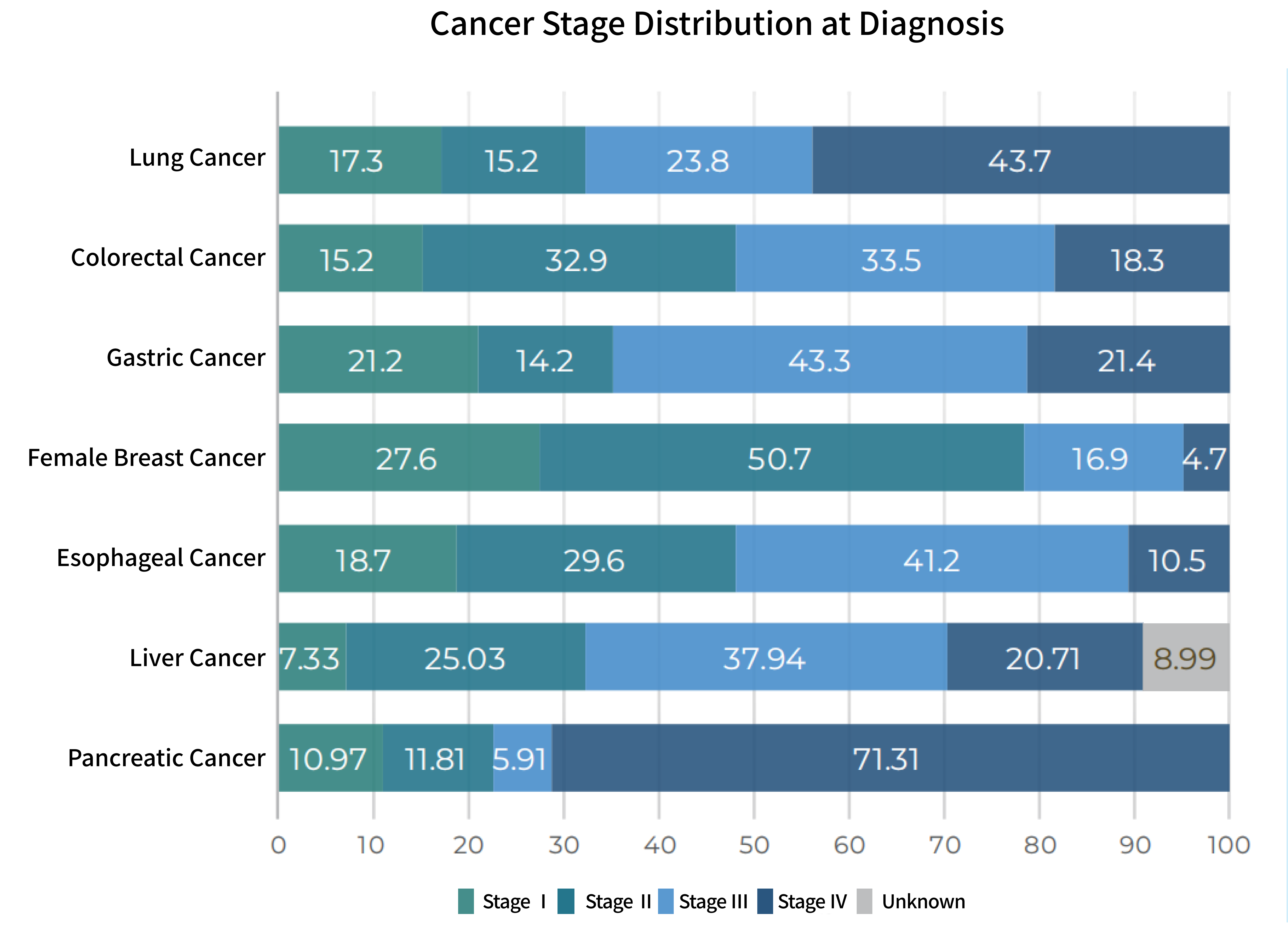
Most cancers are diagnosed at advanced stages, when treatment options are limited and outcomes are poor.
current screening methods often rely on imaging or invasive procedures, which may cause discomfort and lead to patient reluctance.
As the limitations of traditional screening become increasingly apparent, blood-based early cancer detection is emerging as a promising new approach. By capturing subtle cancer signals through a simple blood draw, it overcomes the invasiveness and organ-specific constraints of conventional methods.
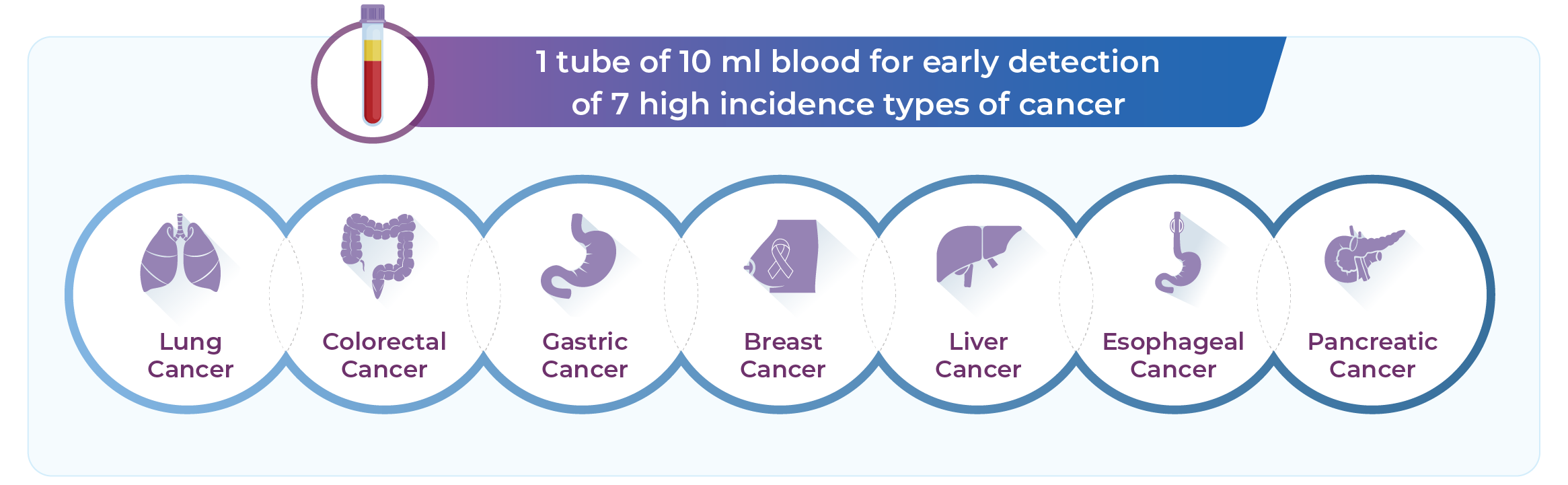
THEMIS takes this a step further—enabling simultaneous screening for multiple high-incidence cancers with a single blood sample, offering a new level of accessibility and confidence in early detection:
THEMIS = Safer + Broader Coverage + Earlier Detection + Higher Confidence
What Can THEMIS Do for You?
General Practitioners
1、Perform multi-cancer screening with just a simple 10ml blood draw
2、Ideal for routine health checkups and initial risk assessment in general practice
3、No expensive equipment needed—easy to implement in outpatient settings
Oncologists & Tertiary Hospital Specialists
1、Support early detection in high-risk individuals
2、Access multimodal cfDNA data including methylation, fragmentation, and CNAs
3、A valuable tool to complement imaging and pathology in diagnostic workflows
Individuals & Families
1、Gain a deeper understanding of personal or family cancer risk
2、Detect early cancer signals during routine health assessments
3、Non-invasive and pain-free
The Four Signals Behind THEMIS
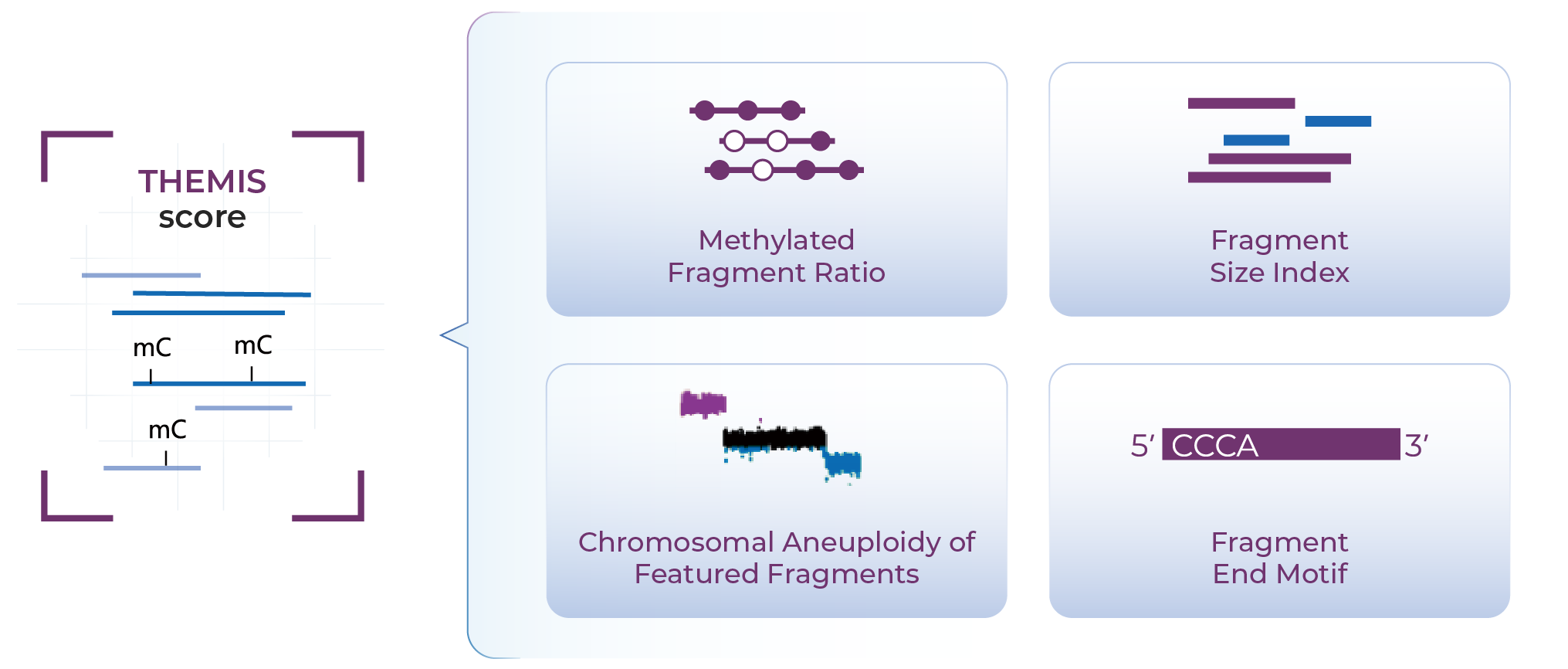
Who should consider using THEMIS for early screening tests?
Adults aged ≥40 years at average risk, especially those between 40–75
Asymptomatic individuals with interest in health monitoring
Current/former smokers with ≥20 pack-year history or recent quitters
Individuals with a first-degree family history of cancer
Patients with cancer-associated infections (e.g., HPV, HBV, H. pylori)
Those with lifestyle-related risks (e.g., heavy drinking, obesity, poor diet)
Patients aiming for early detection to improve long-term outcomes
Screening recommendations above are based on guidelines from the following authorities, and apply to average-risk, asymptomatic adults:
American Cancer Society (ACS)
U.S. Preventive Services Task Force (USPSTF)
American College of Physicians (ACP)
Data validated by a peer-reviewed study published in Nature Communications
Overall detection accuracy (AUC): 0.966
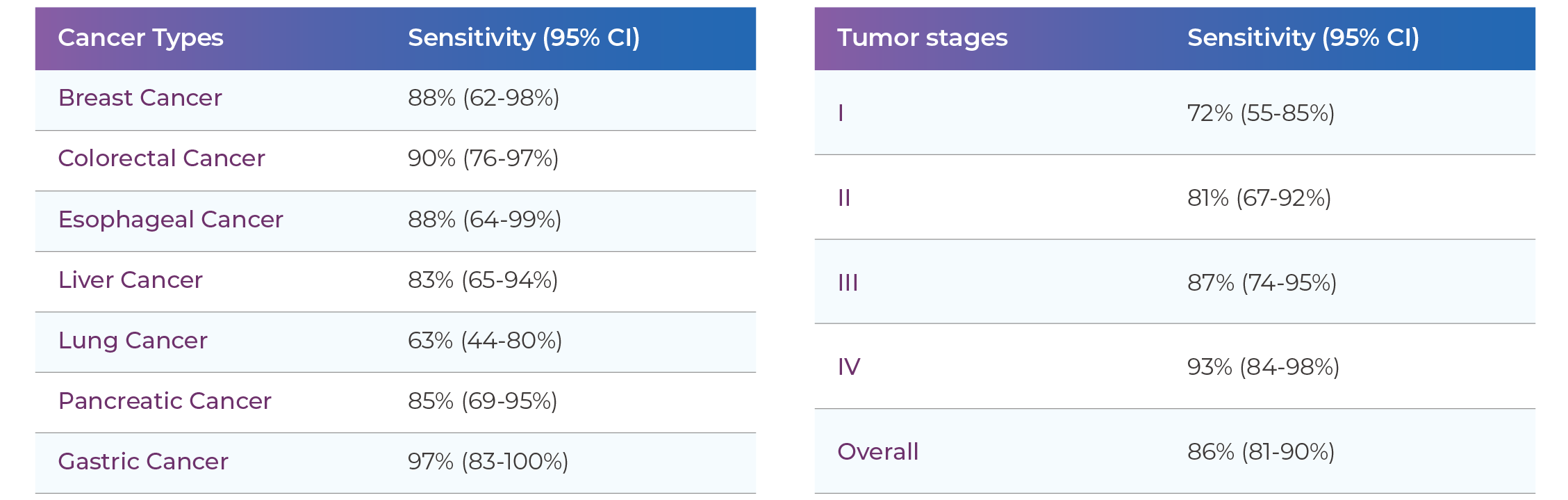
Early-stage cancer (Stage I-II) sensitivity: 73%, specificity: 99%
Covers 7 common high-incidence cancer types
Cancer tissue-of-origin localization accuracy up to 65%
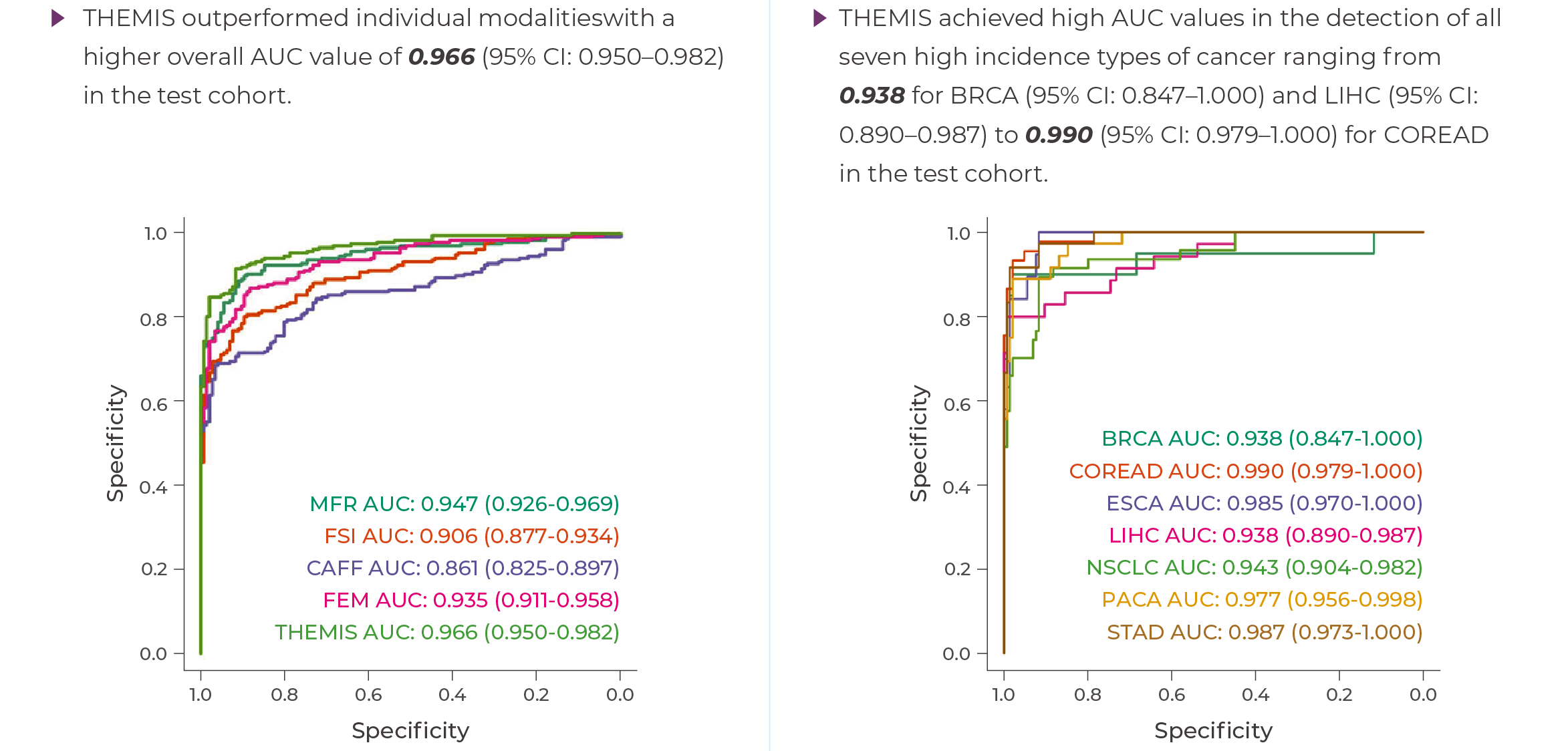
Why Choose Genecast THEMIS?
For Physicians:A clinically validated tool to aid risk assessment and improve early cancer detection:
Improve early cancer diagnosis rates: THEMIS uses multi-modal cfDNA analysis with sensitivity up to 83%, helping you detect cancer signals early and improve patient outcomes.
Reduce false positives: With specificity reaching 99%, it minimizes unnecessary invasive procedures and patient anxiety.
Cover multiple cancer types: One test screens for 7 common high-incidence cancers, saving time and clinical resources.
Provide tissue-of-origin information: Accuracy up to 65%, offering critical guidance for subsequent diagnosis and treatment planning.
For At-Risk Individuals:A safe, non-invasive, and accurate cancer screening experience designed with your peace of mind in mind.:
Non-invasive blood test that avoids pain and risk, making sampling easy and comfortable.
Early cancer detection for better treatment opportunities, significantly increasing chances of recovery.
High accuracy for peace of mind, reducing worry from false alarms and unnecessary follow-ups.
Comprehensive multi-cancer screening in one test, saving time and effort while enhancing coverage.
Understanding Your Genecast THEMIS Results
Negative Result: No tumor markers detected
No tumor-associated markers were found in your blood sample. Please note that the Genecast THEMIS test provides a snapshot of your blood at the time of collection and does not predict future cancer risk.
Next Steps
We recommend continuing with annual Genecast THEMIS testing or other screenings as advised by your physician. If you experience any signs or symptoms suggestive of cancer, please seek medical advice promptly to avoid delayed diagnosis.
Positive Result: Tumor markers detected
Tumor-associated markers were detected in your blood. The result may include predictions of 1-2 potential tumor tissue origins, which can help guide further diagnostic evaluation.
Next Steps
As a screening tool, a positive Genecast THEMIS result requires confirmatory imaging or diagnostic tests to verify the presence of cancer. Please follow your physician’s recommendations for subsequent examinations.
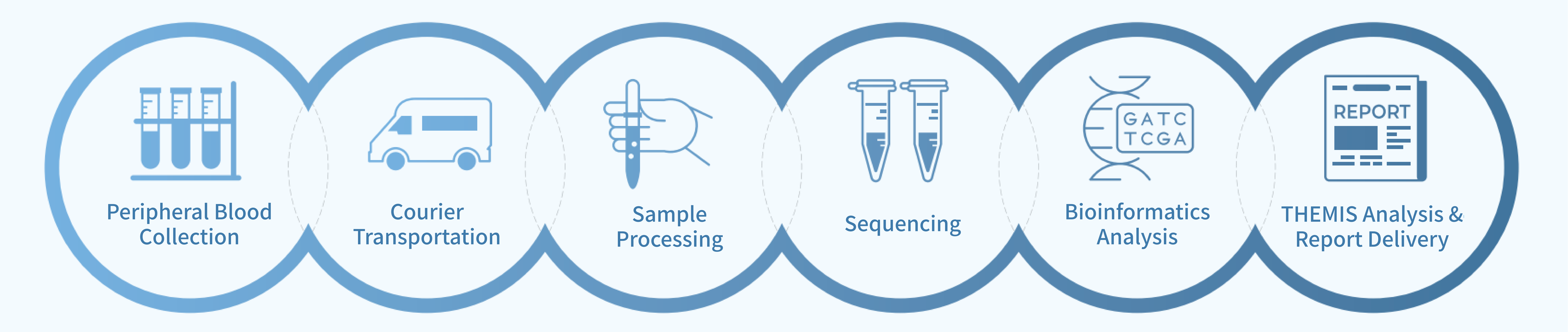
Testing Process
TAT time:20 business days*
(*) Turn-around time takes effect upon sample arrival at Genecast Singapore lab and clears sample quality checks.
You can request the test through your primary care doctor with a simple prescription and a 10ml blood draw.
We use advanced next-generation sequencing to analyze multiple features of the DNA found in your blood plasma.
You’ll receive your detailed ctDNA test results within 20 working days after your sample arrives at Genecast Singapore lab.